Anthony Hyman (2022):
Condensates – Cell droplets as biochemical minilaboratories
In 2009, the British cell biologist Anthony Hyman and his team discovered—while studying unicellular embryos of a pin- or threadworm—a completely new state of biological matter: proteins can gather in high concentration locally in the intracellular fluid. These “condensates” resemble tiny drops that are subject to, among other things, the laws of biophysics. In contrast to other organelles, condensates are not surrounded by an isolating membrane. The strongly elevated concentration of protein inside them provides the stimulus for biochemical reactions that would not be possible outside them. Condensates are formed dynamically, sometimes within seconds, and they usually also dissolve rapidly. During disturbances of their dissolution, which are often age dependent, toxic materials can be deposited in the affected cells, which can trigger degenerative diseases such as ALS or Alzheimer’s. Hyman is searching for new medications that could heal these diseases.
Condensates – Cell droplets as biochemical minilaboratories
Text: Claus-Peter Sesín
Photos: Friedrun Reinhold
Although research into condensates—kicked off by Hyman’s ground-breaking discovery in 2009—is still in its infancy, it is now the fastest growing pioneering field in cell biology. Condensates hold the key to some of the biggest unanswered questions in this field. Researchers in hundreds of laboratories around the world are trying to find leads to understanding the secrets of the complex molecular interactions in the droplets. Pharmaceutical research is also heavily involved—in the hope of being able to use drugs to influence condensate formation and to cure diseases such as Alzheimer’s or ALS (amyotrophic lateral sclerosis). The cause of disease in each case is condensates that have congealed into toxic deposits.
Anthony Hyman was born in the Israeli city of Haifa. After studying zoology at University College, London, he earned his doctorate at King’s College Cambridge in 1987 on embryonic cell divisions of the nematode Caenorhabditis elegans. He then went to the University of California in San Francisco as a postdoctoral researcher. In 1993, Hyman became a group leader at the European Molecular Biology Laboratory in Heidelberg. In 1999, he was one of the founding members of the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) in Dresden, which he continues to head together with a team of directors. He has been a Fellow of the British Royal Society since 2007, an international member of the American National Academy of Sciences since 2020, and a member of the National Academy of Sciences Leopoldina since 2021.
Hyman likes to use the example of a village to explain the scientific significance of condensate research. The villagers work in different places, such as in a bakery or a produce shop. Cells are organized in a similar way. The proteins and RNA of a cell work together in certain areas of a cell, such as the condensates, where they carry out different functions. “To understand these functions, cell scientists have concerned themselves in the last hundred years primarily with individual molecules, but hardly with their collective properties,” says Haman. “That is as if scientists attempting to understand the village would only study the individual behavior of residents, not however the social rules of collective life.”
Images:
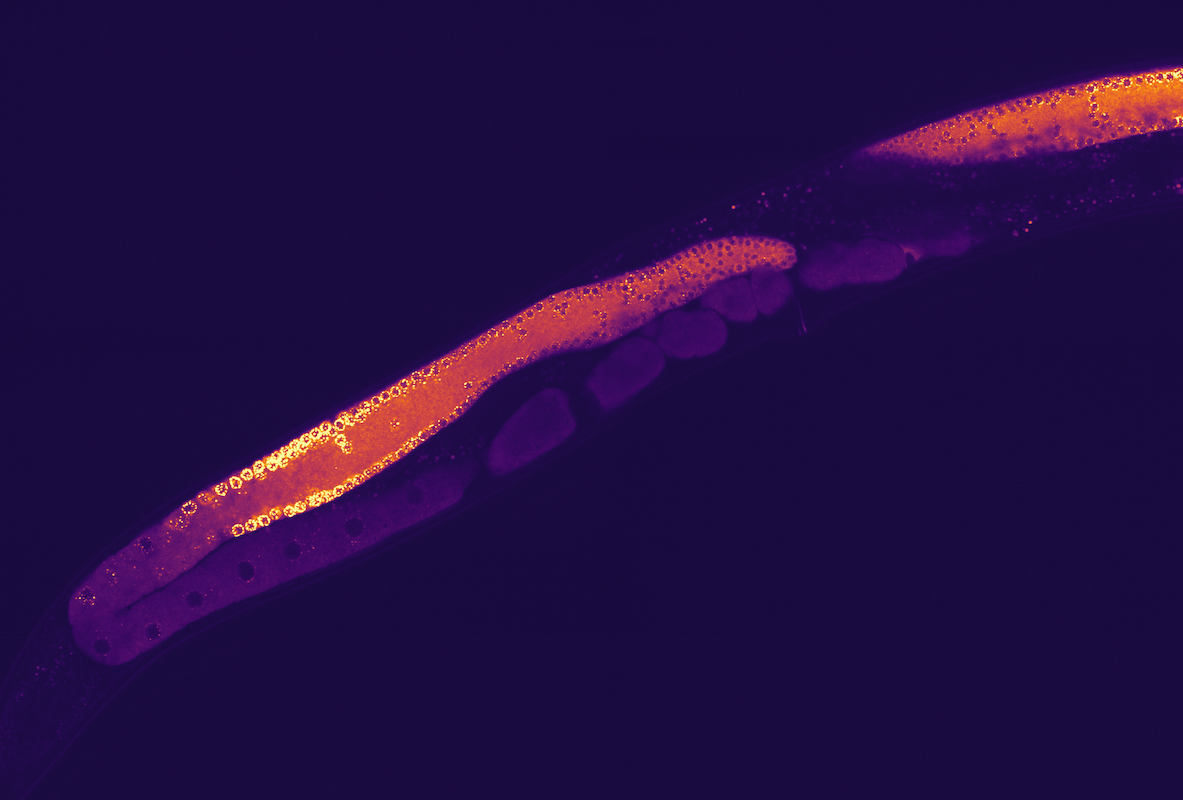
(1) Researchers like to use the one millimeter long pinworm C. elegans as a model system for studying the function and behaviour of membraneless condensates inside an organism. In 2009, Hyman and his colleagues demonstrated that P granules—an important mixture of RNA and proteins in unicellular embryos (red circle) in this worm that is important for its reproduction—do not have a membrane and behave like liquid condensates. Shortly before the asymmetrical cell cleavage of the embryos, the P granule condensates gather in one of the two resulting daughter cells.
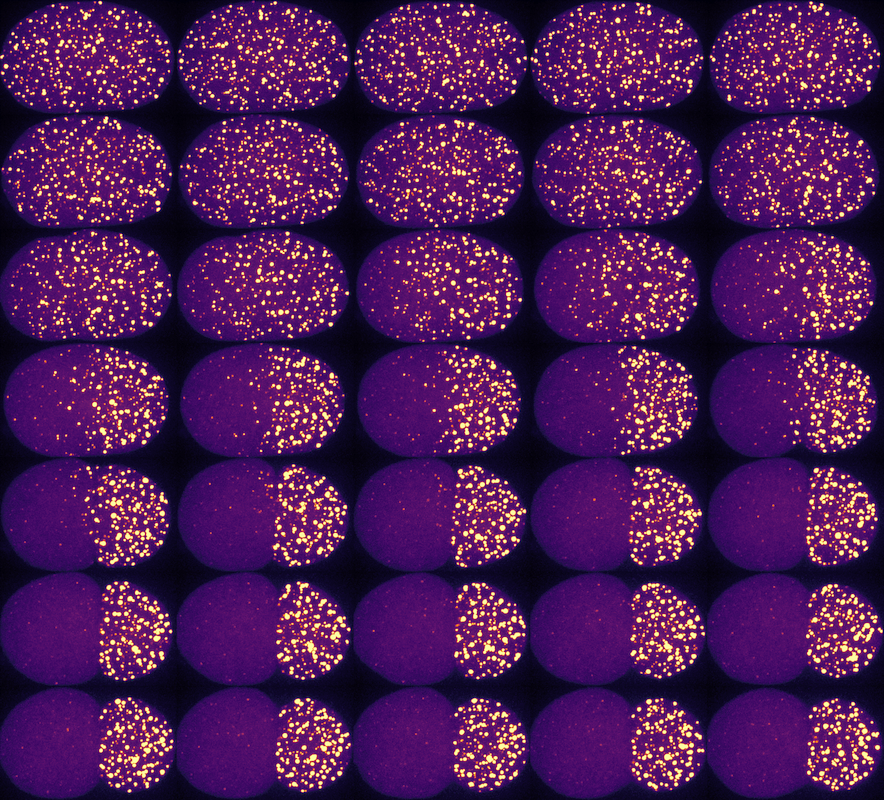
(2) The sequence of images shows the temporal process of the depositing of P granule condensates during the initial cell cleavage of a C. elegans embryo. At first, the P granules are homogenously distributed. Later, they gather on one side of the embryo cells, followed by asymmetric cell division. An important discovery by Hyman’s team was that this process was driven by condensates, and not by direct transportation.
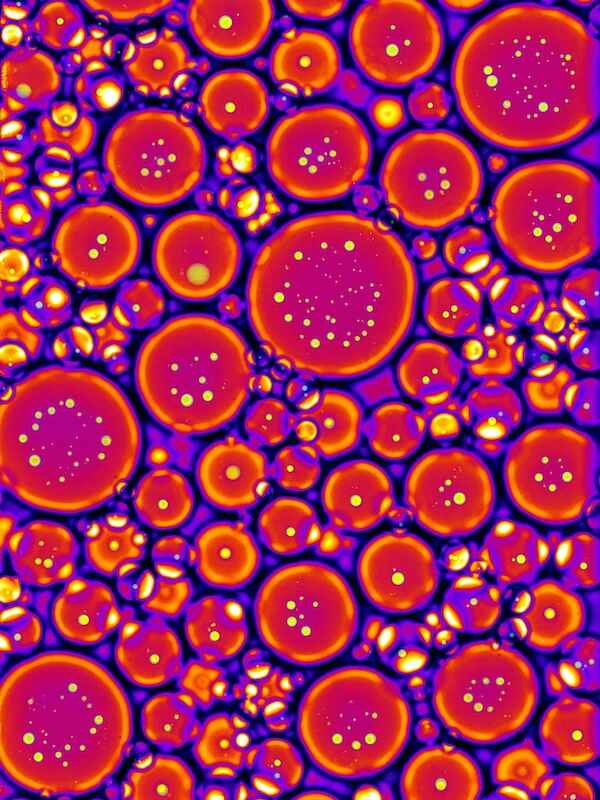
(3) The Hyman team was also able to study condensate formation in a test tube, as shown here in a purified solution of protein in which phase separation was completed. The composite image shows an emulsion of water and oil, which contains fluorescently marked condensates of a P granule protein. Such in vitro studies make it possible for the team to examine phase separation behaviour in a more controlled environment than in unicellular worm embryos.
Interdependence of Biochemistry and Physics
Correspondingly, Hyman’s approach to research is more complicated than a traditional one. While the molecules in condensates interact biochemically, as is customary, the droplets are also subject to the laws of physics. They are formed during what is called a phase change, similar to when water turns into ice in the cold. The increased protein concentration in the condensates—up to one hundred times as high—means that the density of the condensates is also increased. Their behavior is then similar to that of droplets of oil in vinaigrette. Oil and vinegar cannot be completely mixed together. If you let such a dressing stand, the oil droplets that were separated by the energy from stirring then combine bit by bit to form larger drops, until after a couple of hours all of the oil is at the top. The cause of the formation of oil droplets is that oil molecules feel particularly attracted by others like themselves. Their affinity to one another is stronger than that between molecules of vinegar. The difference in affinity drives the phase separation.
A similar observation led to the discovery of condensates in 2009. The physicist Clifford Brangwynne, who was then in Hyman’s team at the Dresdner Max Planck Institute, was examining single cell embryos of the pinworm Caenorhabditis elegans. Prior to an embryo’s first division, P granules form in the intracellular fluid, and according to the academic opinion of the time P granules consisted of thick clumps of protein and RNA. Brangwynne however determined that these P granules behaved more like runny honey. They joined together and then partially separated again, similar to the swirling wax in lava lamps in the 1970s. Just shortly prior to the asymmetric division of the ovum, the P granules localize as a denser mass to one side. Brangwynne and Hyman concluded from this that P granules are liquid and, as condensate, represent a fundamental new state of biological matter. In fact, they soon also identified such condensates in many other organisms.
Hyman and his interdisciplinary team subsequently developed an entire arsenal of methods for observing condensates and for better understanding their function. “We combine concepts from molecular biology, physical chemistry, and soft matter physics,” the Prize winner explains. Even the terminology of this multidisciplinary approach presents a challenge. Each of the different areas of science has its own language. Sometimes they employ different expressions to say the same thing, and sometimes they employ the same expression for different things. “It is a difficult but fascinating problem,” says Hyman. “Our goal is to identify the connection between the collective properties of liquids—such as viscosity and surface tension—and the interaction between individual molecules.”
Hyman also refers to condensates as complex liquids: “It begins with the fact that proteins diffuse freely in the intracellular fluid. Then there is a signal that stimulates the phase separation. This is when proteins separate out of the aqueous medium and form a liquid of their own.” Furthermore, the chemical-physical conditions have to be right: Whether condensates are formed depends, among other factors, on the pH values (degree of acidity), the salinity, and the temperature.

“There is basic agreement in medical research that a fundamental understanding of the causes of diseases is often missing.”
Anthony Hyman
Cellular Division of Labor: A Driver of Evolution
Why are condensates formed at all in cells, how do they arise, and which functions do the serve? To understand this, it is necessary to make a digression into the early history of evolution. The first living beings arose in the primordial ocean some 3.7 billion years ago; according to the conventional understanding, these were unicellular organisms similar to bacteria. These “prokaryotes” already had an external cell membrane but not a cell nucleus of its own. It was only later in the course of evolution—presumably by the symbiotic merger of two (or more) of these primordial cells—that complexer cells arose with their own cell nucleus, the eukaryotes. Eukaryotic cells can be found in all plants and animals.
The cell nucleus that arose in eukaryotes forms a new subdivision (a compartment) in a cell. It is enclosed in its own membrane, so that the processes inside it, such as the reading of DNA, are protected against disturbances from other processes in the cytoplasm. Many other organelles also possess their own external membranes, such as mitochondria, called the power plants of the cells.
It was in this way that a spatial division of labor arose in cells, similar to in Hyman’s village model in which the residents pursue their different respective activities. Without this division of labor it would have been impossible for complex organisms (multicellular organisms such as animals and humans) to even develop during evolution. Eukaryotic cells accomplish more, can handle more tasks at one time, and metabolic reactions are accelerated.
A very rapid reaction is important, for example, if cells are to be protected against environmental stress such as the sudden deprivation of nutrients or falling temperatures. The formation of condensates can then lead to a shutdown of the entire cell activity. This is a survival mechanism for the cells; they survive the stress in a solidified state.
Condensates’ Most Important Feature: Rapid Reactions
Condensates augment this diversity by adding a vital express service. While organelles such as mitochondria are present permanently and work, condensates are formed as a rule spontaneously as needed. This sometimes happens during shock in a matter of seconds. And when they have finished their immediate task, they are usually rapidly dissolved once again. Condensates do not have their own external membrane to isolate themselves from the cell environment. They use phase separation instead.
The most important tool for such a separation is the surface tension that is created between the condensates and the intracellular fluid. The effect can be explained by referring to a pond of water. The surface tension of the water ensures that water fleas can run over the surface of the pond without sinking. It is as if there is skin on the pond. Similarly, the “skin” of the condensate works as a kind of provisional membrane.
Condensates thus function as biochemical minilaboratories that appear spontaneously. Because of the spatial separation of condensates from their environment, it is possible for high concentrations of proteins to gather very rapidly, which either are the prerequisite for some biochemical reactions or accelerate them. Furthermore, condensates can slow down reactions in their vicinity precisely because they deprive the reactions of reactive proteins. The concentration gradient is maintained by the phase separation.
Hibernating Cells
A very rapid reaction is important, for example, if cells are to be protected against environmental stress such as the sudden deprivation of nutrients or falling temperatures. The formation of condensates can then lead to a shutdown of the entire cell activity. This is a survival mechanism for the cells; they survive the stress in a solidified state. “The cells then go into a different material state and become rigid,” according to Simon Alberti, a team leader at the Max Planck Institute until 2018 and since then a professor of cellular biochemistry at the Technical University of Dresden. “This keeps the cells from being damaged. As a rule, this is a temporary deactivation for a subsequent reuse. Similar mechanisms may also play a role in hibernating mammals such as bears.” This has not yet been tested however. Yeast cells and ameba that Alberti’s Max Planck team deprived of nutrition formed a gelatinous mass, and the surroundings acidified. The team also succeeded in solidifying these cells artificially (without a deprivation of nutrition) by lowering the solution’s pH value.
Normally there are many control mechanisms active in cells at the genetic level—by means of what are called regulative proteins. These proteins often have to be especially produced when needed. Signals are then sent to the cell nucleus, where a DNA reading is set in motion. The mRNA that is created has to be transported back into the cytoplasm, where finally the necessary protein is produced in one of the ribosomes (the cell’s protein factory). This takes a relatively long time, in many cases taking too long for spontaneous reactions. Condensates in contrast form within seconds.

Deposits from Condensates Make One Sick
The greatest breakthroughs from condensate research are anticipated in the field of medicine. Many degenerative diseases are caused by proteins that have assumed a wrong structure and consequently are irreversibly solidified. In Alzheimer’s this leads to the deposits typical for the illness. As a consequence, nerve cells in the brain die, and the brain can shrink by up to 20 percent. Alzheimer’s is the most common form of dementia. Every fifth person over 80 years of age is afflicted by it, and a cure is not in sight. Another example is amyotrophic lateral sclerosis (ALS), also incurable and a chronically progressing degenerative disease of the nervous system. ALS is triggered by deposits that form above all in motor nerve cells. This leads to progressive loss of muscle in the arms and legs, an impaired ability to speak and swallow, and respiratory problems. A prominent ALS patient was Stephen Hawking, the British astrophysicist who died in 2018.
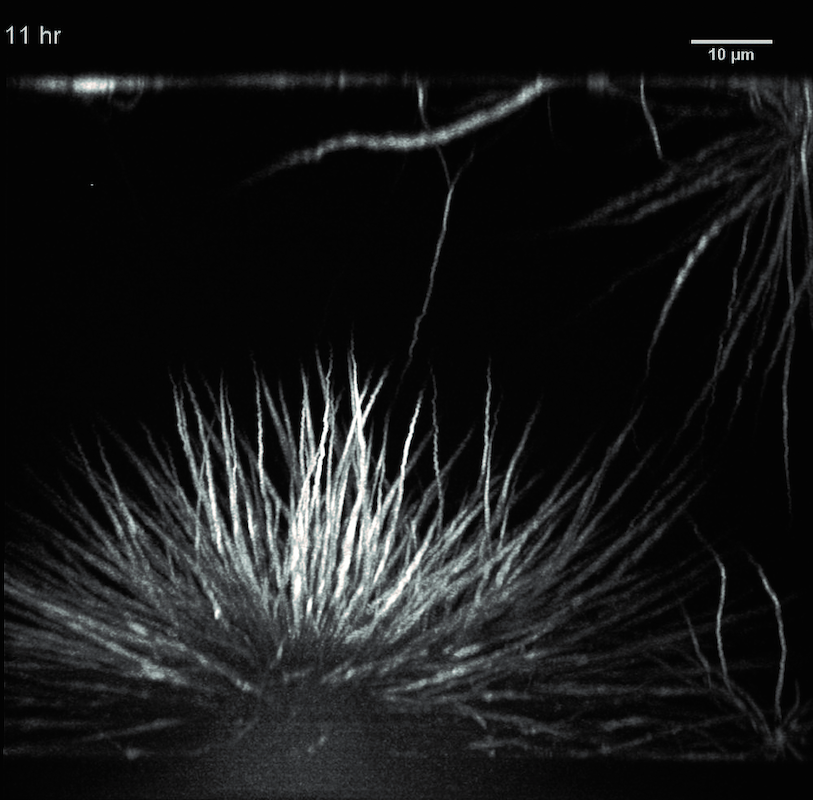
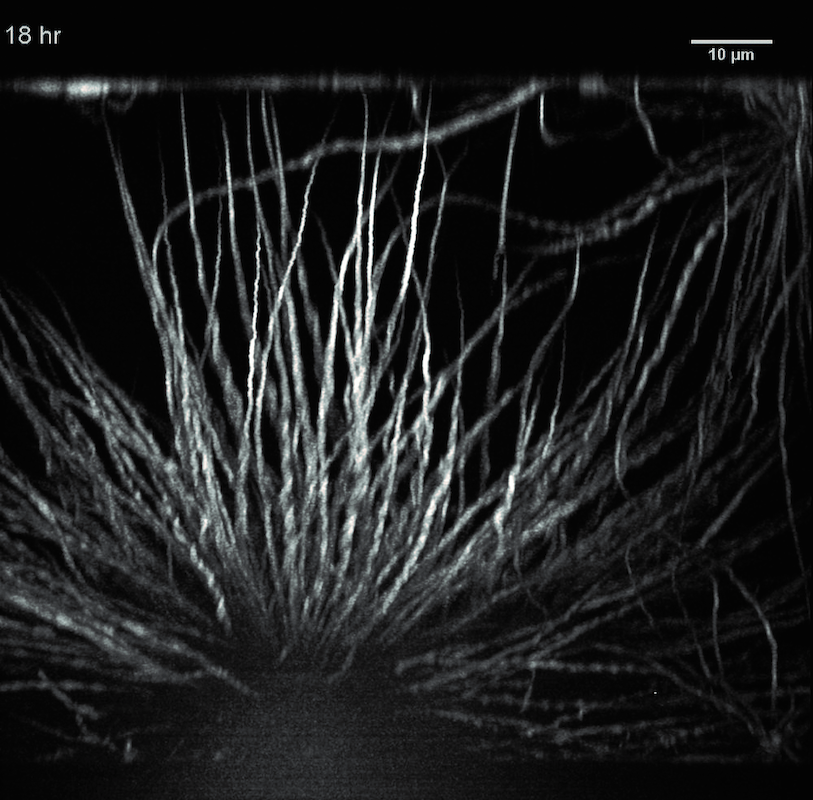
Such toxic deposits often occur because proteins have undergone pathological changes in old age. Hyman and his team have studied the FUS protein extensively, whose mutated forms very probably trigger ALS. “We do not yet know the precise function of FUS,” says Hyman. “FUS is localized in the cell nucleus and has something to do with DNA repair.” In healthy cells FUS normally forms liquid phases, but in ALS patients it takes on more rigid or rigid forms. If proteins stiffen to a gel or crystal, the chemical reactions in them come to a standstill. The chances then sink that they might revert to a liquid phase. If the FUS proteins are pathologically modified, then the deposits are often permanent.
In Alzheimer’s disease, the TAU protein—which primarily appears in nerve cells—is involved in the disease’s origin. It forms the amyloid deposits typical for Alzheimer’s. “We have studied TAU proteins in a test tube and seen that the liquid phase has been transformed into a solid form after a few hours,” Hyman reports. “We have also looked at a variant with a pathologically modified amino acid sequence, and in it the phase transition went faster than in the normal variant. It could thus be that many neurodegenerative diseases are triggered by proteins that actually do not play a very large role in the normal physiology of cells, but do cause problems when they are organized incorrectly.”
Time series of a protein condensate during the transition from liquid to solid. The protein FUS (fused in sarcoma) is connected to neurodegenerative diseases.
Novel Therapies for Neurodegenerative Diseases
Hyman may have discovered a key molecule that is capable of dissolving condensates that are already formed. This is adenosine triphosphate (ATP). This molecule is released by mitochondria and provides living cells with the largest portion of the energy they require. Members of Hyman’s team at the Dresden Max Planck Institute gave ATP to protein condensates and determined that the condensates then disappeared. This even occurred when the protein concentrations in the condensates were very high. This discovery is interesting especially because “the ATP production in old age can decline due to malfunctioning of mitochondria,” according to Hyman. “It is possible that modulation of the ATP level can be used in order to alleviate degenerative diseases of aging that accompany toxic condensates.”
“In most cases we still do not know precisely what drives the formation of condensates and which function they have,” Hyman says. “The problem is that the dynamics of protein formation and interactions must also be taken into consideration in structural studies.” Hyman expects “great advances in our understanding” within the next five years. One goal he has set himself is to decode the “molecular grammar” of condensates—which means identifying the amino acid codes that influence the biophysical behavior of proteins. “Today we already know that the amino acid glycine ensures that condensates remain fluid, while the amino acids glutamine and serine expedite solidifying.”
Hyman wants to utilize the funds that accompany the Körber Prize to refine his study methods even further. One of the largest challenges is to track the formation of condensates in real time. The Prize winner is convinced “that our cell biological understanding of condensate formation will exert an important influence on the future development of medication.” For this reason, Hyman was also a cofounder of the company Dewpoint Therapeutics, located in Boston and Dresden. One objective of its work is to study the effect of medication on condensates. As its top priority, the firm is working on identifying the appropriate medicines to block the formation of the deposits that lead to diseases. Also to be studied is the extent to which a large number of condensates in a cell impairs the effectiveness of existing medicines. “There is basic agreement in medical research,” according to Hyman, “that a fundamental understanding of the causes of diseases is often missing.”

Condensates: The Primordial Form of All Life on Earth?
Condensate research is also throwing new light on the origin of life on our planet. Researchers at the Max Planck Institute of Molecular Cell Biology and Genetics and at the Max Planck Institute for the Physics of Complex Systems have come to the result that the very first living organisms in the primordial ocean could have taken the form of condensates, not cells. According to this understanding, condensates were the precursors of the prokaryotic primordial cells. The Max Planck teams have determined experimentally that condensates are capable of dividing like cells. In the process, condensates have apparently also acquired the property of being able to pass on genetic information. It was only later that the droplets formed external membranes and thus taken on the form of the first primordial cells.
Macromolecules have probably been constantly created in the primordial ocean. As they became able to conduct phase separations, they acquired the form of condensate droplets that attracted one another and merged. “Biochemical reactions could have taken place in these droplets that would not have been possible outside them because the concentrations in the primordial ocean were not high enough,” said Hyman. “If this is correct, then condensates are relics from a primordial time that have managed to survive in cells until today, similar to flies in amber.
The prizewinner

Anthony A. Hyman was born in Haifa in 1962. His father, a British computer engineer, was employed in Israel. His mother was a painter. Anthony was still a small child when the family returned to England. “I was a very dreamy child and never thought about a career, except as a bicycle mechanic.”
Even while he attended school nothing pointed to Hyman’s genius. “I had bad grades and my results on tests were never good.” After completing school, Hyman took a position as a laboratory assistant at the University College London (UCL), where among other tasks he prepared cell cultures for scientific studies. The work fascinated him, and following a recommendation by the biologist Terry Preston he soon undertook his own small experiments. Sometimes he sat in the lab until midnight. Preston advised him to study biology.
Hyman studied biology at the UCL, which belongs to Cambridge University, and specialized in cell biology. In 1988[MW1] he was awarded a Ph.D. for work on embryonic cell division in the pinworm Caenorhabditis elegans. After completing his graduate studies, Hyman went to the University of California in San Francisco, USA, for five years, where he worked on internal cell structures (microtubules). In 1993 Hyman started as a group leader at the European Molecular Biology Laboratory in Heidelberg. A big influence on him there was the French cell and molecular biologist Eric Karsenti: “Karsenti was convinced that it was only possible with the help of physics to understand the dynamic processes in a cell.” In 1999 Hyman was one of the founding members of the Max Planck Institute of Molecular Cell Biology and Genetics in Dresden, which he heads together with a team of directors. Since 2007 he has been a Fellow of the British Royal Society, since 2020 an international member of the American National Academy of Sciences, and since 2021 a member of the National Academy of Sciences Leopoldina.
In 2009 Hyman and his team discovered condensates—tiny droplets in the intracellular fluid, which function as minilaboratories which make spontaneous biochemical reactions possible. Condensates constitute a completely new material state of living matter and are subject to, for example, the laws of biophysics. Hyman was married to the American microbiologist Suzanne Eaton (1959-2019), who have two sons. In his free time, he enjoys cycling as well as playing “the flute whenever he has time, mostly classical music and preferably Bach.”
Interview with Tatjana König
Tatjana König about the Körber Prize

“On the other hand, it is important precisely now to strengthen a European science that stands on the foundation provided by freedom and democracy.”
Tatjana König
Former member of the Executive Board of Körber-Stiftung
Why does the Körber Foundation award a European prize instead of a national or an international one?
Even in 1985, when he endowed the Prize, European rapprochement und agreement were Kurt A. Körber’s core concern. It was clear to him that a prize for all of Europe, west and east, would have a special symbolic meaning in a world of international conflicts and national egoisms—at the time the iron curtain still seemed practically insurmountable. The political developments of the past years and, above all, the events since the beginning of 2022 demonstrate clearly to us that we unconditionally have to hold on to the mission of unifying Europe, which is underscored by a science prize for Europe.
Have recent events not given a massive dampener to the idea that science can serve as an element for improving international understanding?
On the one hand, yes, because it makes clear that even science does not operate in politically neutral space and that we in the future have to examine much closer which scientific collaboration we want to enter into, to which purpose, and with whom as well as whether it is acceptable. On the other hand, it is important precisely now to strengthen a European science that stands on the foundation provided by freedom and democracy. It is only on the basis of this self-understanding that we will be able to achieve genuine innovation. Above all, it is only in this way that we will be able to defend our values.
Which role can the Körber Prize play in this situation?
Collaboration is essential in science. Scientists—female and male—from all of Europe and the world are working at Europe’s research institutes, and the research groups are diverse and international. Without this cross-border exchange of knowledge Europe would one day be left behind as a site for innovation and pioneering scientific knowledge. For these reasons the Körber Prize very intentionally promotes basic research in Europe, in order for it to be able to keep pace in the future in the competition for the best ideas. Our goal is to bring together the European scientific community at the occasion of the presentation of the Körber Prize in order to identify the parameters and challenges for European science to be successful.
A word about this year’s Prize winner?
For all of our Prize winners, we can hopefully demonstrate what an important contribution science can make towards coping with great challenges, even and especially when this science is taking place at the stage of basic research. Anthony Hyman is another wonderful example of this. As a British citizen he has lived and worked for many years in Dresden, where he heads an international team. His discovery of cell condensates, a fundamentally new state of biological matter, is basically like stepping onto a new planet. While we still do not know what we will encounter there, we can be sure that, for example, our understanding of neurodegenerative diseases will be raised to a new level and will ideally open entirely new pathways to healing them.
Awards Ceremony 2022

Admission in the foyer of Hamburg City Hall before the award ceremony Photo: Körber-Stiftung/Claudia Höhne 
From the left: Tatjana König (Member of the Executive Board of the Körber-Stiftung), Prof. Dr. Martin Stratmann (President of the Max Planck Society), Prof. Dr. Anthony Hyman (Körber Prize winner 2022), Dr. Peter Tschentscher (First Mayor of the Free and Hanseatic City of Hamburg), Dr. Lothar Dittmer (Chairman of the Executive Board of the Körber-Stiftung) Photo: Körber-Stiftung/Claudia Höhne 
Dr. Peter Tschentscher welcomed the guests to the presentation of the Körber Prize 2022. Photo: Körber-Stiftung/Claudia Höhne 
Around 500 guests were invited to the award ceremony. Photo: Körber-Stiftung/Claudia Höhne 
In his speech, Dr Lothar Dittmer referred to the vision of the founder Kurt A. Körber to award science that promises to make a significant contribution to the preservation of living conditions on our planet. Photo: Körber-Stiftung/Claudia Höhne 
Dr. Helen Czerski during her talk with Prof. Dr. Anthony Hyman Photo: Körber-Stiftung/Claudia Höhne 
Dr. Helen Czerski in conversation with Tatjana König and Prof. Dr. Martin Stratmann Photo: Körber-Stiftung/Claudia Höhne 
The scholars of the Deutsche Stiftung Musikleben, Fabian Egger (flute) and Johann Zhao (piano), provided musical entertainment. Photo: Körber-Stiftung/Claudia Höhne 
Dr. Lothar Dittmer, Prof. Dr. Anthony Hyman and Prof. Dr. Martin Stratmann after the presentation of the certificate Photo: Körber-Stiftung/Claudia Höhne 
Katharina Fegebank (Second Mayor of the Free and Hanseatic City of Hamburg) and Prof. Dr. Anthony Hyman Photo: Körber-Stiftung/Claudia Höhne 
Reception after the presentation of the certificate Photo: Körber-Stiftung/Claudia Höhne
Photos of the presentation of the Körber European Science Prize 2022 to Anthony Hyman in the Hamburg City Hall on 02 September 2022
These photos are free to use in the context of news coverage with the credit Körber-Stiftung/Claudia Höhne.
Cell Droplets as Biochemical Minilaboratories