Alice Whitby
Clare Grey (2021):
New Batteries for more Climate Protection
The British chemist pioneered the optimisation of batteries using NMR spectroscopy. This method provides non-invasive insights into the inner workings of batteries – and is similar to magnetic resonance imaging, which doctors use to screen patients. Her NMR studies helped to significantly increase the performance of lithium-ion batteries, which supply power to mobile phones, notebooks or e-vehicles, for example. Furthermore, Grey was instrumental in the development of new types of batteries – including lithium-air batteries, which have a tenfold increase in energy density, and others that charge very quickly and are particularly safe in operation. She is also conducting research into cost-effective and durable storage systems for electricity from renewable sources. Clare Grey sees her research as an important contribution to achieving the European Union’s declared goal of climate neutrality by 2050.
New Batteries for more Climate Protection
Text: Claus-Peter Sesín
Photos: Alice Whitby
The British chemist Clare Grey has performed pioneering work in using NMR spectroscopy to optimise batteries. This technology has made it possible to non-invasively view the inner workings of batteries, analogous to how doctors employ magnetic resonance imaging to screen patients. Grey’s NMR studies have helped to significantly increase the performance of lithium-ion rechargeable batteries, which supply power to, for example, mobile phones and electric cars. She was, furthermore, heavily involved in the development of novel types of batteries, including lithium-air rechargeable batteries that have the potential to exhibit a tenfold increase in energy density and other types that recharge very quickly and operate particularly reliably and safely. Grey also conducts research on economical and durable storage systems for electricity produced from renewable sources. She views her basic research as an important contribution to achieving the European Union’s declared goal of climate neutrality by 2050.
The size and weight of the first mobile phones were reminiscent of a large building brick. The phones did not become truly “handy” until the beginning of the 1990s with the introduction of lithium-ion rechargeable batteries. This type of battery, relatively light despite being powerful, was originally developed for the first portable video cameras, which with the previously customary nickel cadmium batteries were heavy burdens on the user’s shoulder. Hardly any of the engineers of the time would have imagined that lithium-ion rechargeable batteries would open the door to future portable and internet-capable communication devices, let alone to the current era of electromobility.

“There have been significant advances in lithium-ion rechargeable batteries since 1991. Their energy density has tripled. That means their weight can be reduced by two thirds, while retaining the same electrical storage capacity.”
Clare Grey
The British chemist has made decisive contributions to this development. Grey is a pioneer in using NMR spectroscopy to conduct research on solids. In the process, the resonance of atomic nuclei is used to achieve novel views inside materials such as batteries. With the aid of NMR technology, Grey was able to examine the electrochemical processes that occur during the charging and discharging of rechargeable batteries while they were being used or operated and to follow the movement of lithium ions in and between the various materials in a battery.

An NMR spectrometer in Clare Grey’s laboratory. The electromagnet in it produces a magnetic field that is approximately 260,000 times as strong as that of the Earth. Inside this spectrometer, Grey’s team can, for example, test mini versions of novel lithium rechargeable batteries while they are in operation. 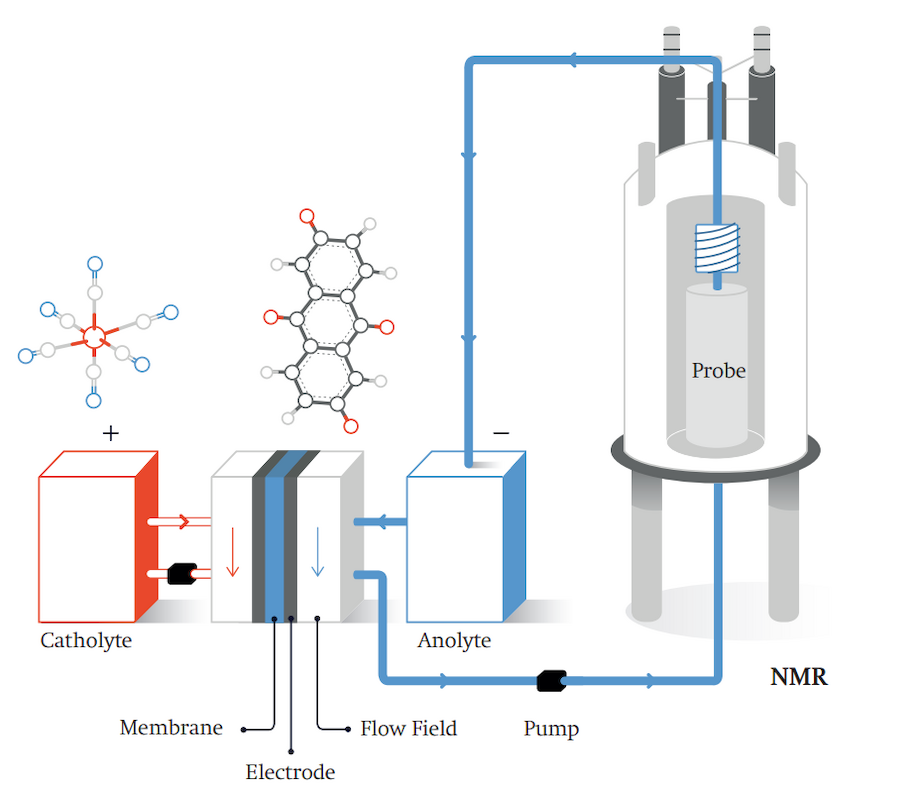
Symbol picture of a novel redox flow battery. This type of rechargeable battery, also known as a flow battery, stores electrical energy in two external tanks of chemical solutions. Redox flow batteries can also be optimised using NMR technology.
Nuclear resonance reveals molecular structrues
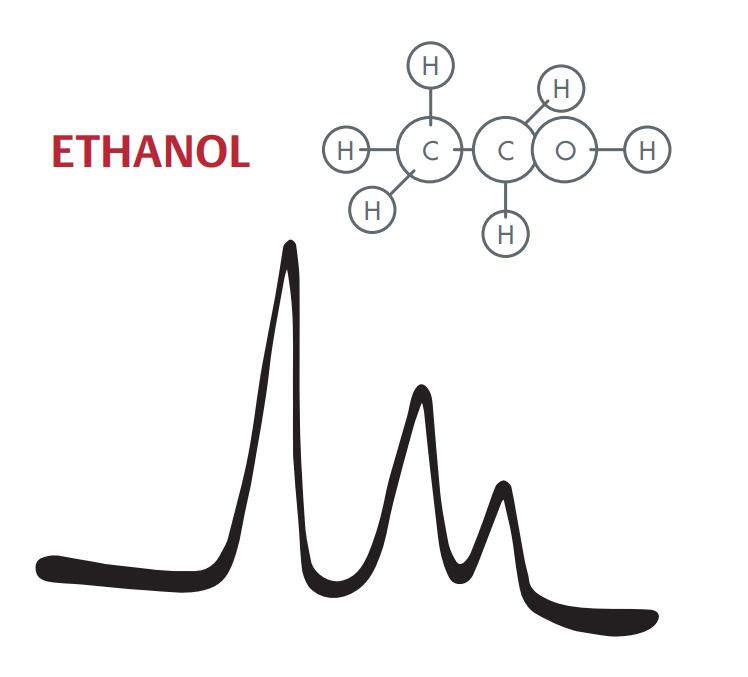
A particularly powerful alternative to this is NMR spectroscopy. The abbreviation NMR stands for nuclear magnetic resonance. Such oscillations of atomic nuclei were studied for the first time by the physicists Felix Bloch and Edward Mills Purcell in 1946. They exposed ethanol molecules to a strong static magnetic field. The field forces certain atomic nuclei—those exhibiting a magnetic moment, such as the nuclei of the hydrogen atoms contained in ethanol—to align according to the magnetic field lines, similar to the needles of a compass in the Earth’s magnetic field. They also radiated the ethanol molecules with radio waves from an appropriately designed coil. They thus determined that the atomic nuclei of hydrogen began to oscillate at certain radio frequencies. Similar to a wobbling toy top, the tips of the hydrogen nuclei performed precision circular movements. For ethanol, Bloch and Purcell identified three resonances that manifested at different frequencies. They were now able to draw an NMR spectrogram. Ethanol exhibited three different high oscillation peaks because the hydrogen contained in the molecule is present in bonds of three different strengths (figure). NMR thus made it possible to also draw conclusions about the structure of the ethanol molecule. Later, physicians used a modified form of NMR technology in magnetic resonance tomographs, in which computers combine the resonance data into images of slices of the human body.
NMR functions most simply with liquid samples in which the molecules move very quickly, which averages out the interactions between them. As one of the first in her field, Grey, in contrast, used NMR to examine solids such as metal oxides. During her stay in the US, she became acquainted with scientists working for Duracell, who encouraged her to employ NMR technology to study the materials in batteries. “The previously customary use of X-rays in examinations only produced an average view of the structure,” Grey said. “By using NMR I was now able to detect the fine details in these often disordered materials, as well.” In the beginning she studied individual materials after opening the batteries at a certain stage of their charging and life cycles. One of her aims was to determine which chemical processes cause the batteries to age and how their longevity and capacity could be increased. Later, Grey improved the NMR technology so that it became possible for her to examine the cells nondestructively while they were functioning, in what are called in situ experiments. These measurements helped her track down transient substances and enormously accelerate the studies.
Grey is a pioneer in using NMR spectroscopy to conduct research on solids. In the process, the resonance of atomic nuclei is used to achieve novel views inside materials such as batteries.
In the usual lithium-ion rechargeable batteries the anode (the negative pole) usually consists of graphite and the cathode (the positive pole) of a metal oxide in which other metals such as cobalt, nickel, and/or manganese are present in addition to lithium. Between the two electrodes there is an organic electrolyte solution, usually ethylene carbonate. When the battery discharges, the positively charged lithium ions move from the graphite anode to the cathode, where they are inserted into the metal oxide. At the same time, an electrical current (free electrons) flows via external connections to, for instance, power an electrical motor. The process runs the other way around when the battery is being subsequently recharged. The lithium ions flow back to the anode and insert into the graphite there. Ideally, these charging-discharging cycles can be repeated thousands of times.

Ein Doktorand aus Greys Team bereitet die Elektrode einer gebrauchten Lithium-Batterie für eine NMR-Untersuchung vor. Die Batterie wurde in einem bestimmten Zustand ihres Lebenszyklus in Einzelteile zerlegt, um Ursachen für Defekte oder vorzeitigen Verschleiß herauszufinden. 
Rechargeable batteries for long distances
In 2015 Grey presented a novel type of lithium-air rechargeable battery, with an anode consisting of metallic lithium and a cathode of porous carbon. During discharging, the lithium ions flow to the cathode, where they, together with oxygen from the air, are transformed into either lithium peroxide or lithium hydroxide. The outstanding feature of the new lithium-air battery is its energy density, which can be as much as ten times higher. Given the same weight, such batteries store up to ten times as much electrical energy, which could help electric cars to reach a range similar to that of cars powered by gasoline. Until now, however, lithium-air rechargeable batteries age too quickly; they survive only a limited number of charging cycles. Grey is also working on the development of other types of rechargeable batteries with high energy densities.

“Electrochemical cells are complex systems in which a multitude of components interact in a fascinating manner. We can only advance this technology if we understand very precisely and in every detail how these materials work and function.”
Clare Grey
Theoretically, it is already possible to build electric cars with today’s lithium-ion rechargeable batteries that can go as far as a car on one tank of gasoline. That, however, would require installing so many batteries that they would occupy a large portion of the car’s interior. Furthermore, electric cars would then be so heavy that they would consume an enormous amount of energy at every start, such as at red lights, just to accelerate this large mass. As a consequence, a car’s range in city traffic would sink noticeably. As a compromise, the manufacturers of electric cars install fewer lithium-ion rechargeable batteries, making the cars relatively light and spacious, but also limiting their typical range to 150 to 400 kilometers before having to recharge. New types of rechargeable batteries with a high energy density, in contrast, could make “gasoline ranges” possible without the burden of additional battery weight.

Battery tests as in NMR Tomography
Among the team’s most important tools are NMR spectrometers. The devices function similarly to NMR tomographs in medicine, but are smaller, and the electromagnet that produces the strong static magnetic field stands upright. The experiments are performed in a probe head or platform inside the electromagnet. Located on it is a small copper or silver coil—arranged horizontally—that produces radio signals to excite the samples. The samples themselves are placed in small glass tubes, zirconia rotors, or in Clare Grey’s case, very small batteries and inserted into the coil.
To be able to produce the extremely strong magnetic field that is 10 to 20 Tesla strong (up to 300,000 times that of the Earth’s), the electromagnet of the NMR spectrometer is equipped with superconducting coils, which have to be kept cold, close to absolute zero (-273.15 degrees Celsius). The NMR spectrometers are located in a secure area of the laboratory because the magnetic field would pull metal parts in its vicinity to it at such a high speed that any individuals in their path could be seriously injured and the magnets destroyed.

Radio messages from the nucleus of atoms
In order to get the atomic nuclei in the probes to oscillate at their respective typical resonance frequencies—which also depend on the respective chemical bond and on neighboring nuclei—the researchers apply a pulse of short high-frequency alternating voltage to the copper coil. This voltage produces a pulsed radio wave in the middle of the coil and is aligned perpendicular to the strong static magnetic field. The radio pulse excites the atomic nuclei over a wide frequency range and causes them to oscillate. As soon as the pulse stops, the small magnetic fields of the atomic nuclei rotate around the static magnetic field of the electromagnet, in the same way that a gyroscope or top rotates once it has been set spinning. The nuclei themselves emit very weak radio waves during this “precession,” which are registered by the copper coil—now working like a radio receiver—and subsequently evaluated on computers. The spectrograms produced in this manner make it possible to draw conclusions concerning the local structures and electronic properties of the samples. Cross-sectional images can even be created, similar to those from a medical tomograph.
The NMR examination method only functions with atomic nuclei that—like a hydrogen nucleus—have a magnetic moment, which are similar to tiny compass needles. The prerequisite for this is that either the number of protons in the nucleus or the number of neutrons (or both) is odd. Some 40 percent of all naturally occurring chemical elements satisfy this condition. Among them is the alkali metal lithium, but not for example the most naturally occurring forms of carbon or oxygen. The nucleus of a carbon atom (atomic number 6) consists of six protons and six neutrons. The nucleus of an oxygen atom (atomic number 8) has eight protons and eight neutrons.

Test apparatus for lithium-air batteries and other types of lithium batteries. 
The team uses this analysis system to study gases that the tested batteries (here a lithium-air battery) absorb or release during operation. 
Lithium batteries are tested here to measure their performance and to detect degradation due to aging.
As a solution, Grey’s team can employ other naturally occurring isotopes such as carbon-13 or oxygen-17, whose nuclei have an additional neutron, but are present in much lower natural abundance. If the samples being studied contain these isotopes, then carbon and oxygen (in this example) will be visible in the NMR spectrogram. The inclusion of isotopes has practically no influence on the chemical properties of the molecules (and thus of the batteries), which are determined almost exclusively by bonds in the external electron shells of atoms
Using the NMR spectrometers, the team studies, for instance, the raw materials for new types of electrodes, but also trial batteries that are millimeter small and whose internal workings can then be inspected while in operation, i.e., in real time. Such in situ studies offer enormous advantages compared to conventional test procedures: “Many of the chemical substances that originate during the charging or discharging of rechargeable batteries are transient but still play a key role in the aging processes,” Grey explains. “Many of these materials will have long disintegrated by the time batteries are cumbersomely disassembled.”
A large manufacturer of mobile phones had to take its newest model off the market in 2016 because of safety issues and safety remains a primary (or significant) concern for both battery scientists and battery users. One such safety problem is the so-called dendrites. These are tiny fir tree-like structures of metallic lithium that can grow out of the lithium battery’s graphitic anode. Dendrites are formed in particular when batteries are charged too quickly, because then the lithium ions cannot be stored in the graphite anode in an orderly fashion. Instead, a metal deposit grows on the anode’s surface.
In the worst case, these “metal saplings” bridge the two electrodes and reach the cathode, which can lead to a short circuit and start a fire. The users demand very short charging times—true all the more so for electrically powered cars— yet the danger of dendrite formation grows if charging proceeds too quickly. “Using NMR technology we were able for the first time to watch the dendrites while they were growing,” Grey said. Such lithium saplings often simply break loose from the anode. Then there is “dead lithium” swimming in the electrolytes, with the consequence that the storage capacity of the rechargeable battery declines with age.

Environmentally friendly high-performance batteries
Above all, Grey is interested in increasing the energy density of rechargeable batteries, reducing charging times (without a danger of fire), reducing the production costs (the current electrically powered cars are still quite costly because of the expensive batteries), and employing raw materials that are as environmentally friendly as possible. To achieve the last of these goals, Grey is also conducting research on batteries for the “beyond lithium“ era, which contain environmentally more friendly metals such as sodium in place of lithium. Sodium batteries are however heavier than lithium batteries—lithium is the lightest of all metals—and are therefore more suitable to serve as less expensive stationary buffer storage for wind- and solar-generated power. Magnesium, from the alkaline earth metals group, can also replace lithium in rechargeable batteries.
Very promising are also new versions of rechargeable batteries whose anodes contain silicon, a metalloid (with properties between metals and nonmetals), in place of graphite. Silicon is very abundant—literally like the sand of the sea—being the second most common element on the planet after oxygen. “Silicon is an extremely important alternative to graphite, because it can store up to ten times more electrical energy during charging, but it is only just starting to be used commercially,” according to Grey. Unfortunately, it has the disagreeable property that it expands up to three times in size. This is, according to the predominant school of thought, the reason for the current poor performance of rechargeable batteries with silicon anodes. Yet with the aid of NMR and X-ray studies, Grey was able to prove that the decisive factor was not the expansion in size per se but chemical reactions of the partially crumbling silicon with the electrolyte, which consume lithium and result in very rapid fading of the battery

“Silicon is an extremely important alternative to graphite, because it can store up to ten times more electrical energy during charging, but it is only just starting to be used commercially.”
Clare Grey
Short circuits can trigger fires
Grey is also working on improving the electrolyte: “The ideal electrolyte conducts ions very well, is chemically stabile, inexpensive, and not toxic.” The importance of electrolytes for the functioning of a rechargeable battery can be seen in the fact that knowledge of their precise ingredients belongs to the best kept secrets of battery manufacturers. Organic salt solutions are most frequently employed as the electrolyte in lithium-ion batteries. There is the threat of significant danger if the battery has a short circuit: “Then the electrolyte can quickly reach temperatures of over 250 degrees Celsius, which can also lead to fires or even explosions,” says Grey. To improve the safety of rechargeable batteries, her team is attempting to replace the existing fluid electrolytes by ones consisting of a solid polymer or ceramic material. A short circuit of a conventional lithium-ion battery can occur, for example, in electric cars in an accident.
In another project, Grey is working on optimising novel redox flow batteries. This type of battery, also known as a flow battery, stores electrical energy in two external tanks of chemicals. These tanks can, in principle, be of any size and can be extended at any time as needed in order to increase the storage capacity. The chemical reactions take place in a galvanic cell, in the middle of which an ion-selective membrane separates the fluids from one another. The two fluids circulate through the system with the aid of pumps. The most common version of this type of battery is the vanadium redox flow battery, in which salts of the element vanadium are dissolved in the electrolyte solutions. In this context, vanadium can assume different stages of oxidation. It is an element that occurs relatively frequently, but is poisonous

Because of their scale and the relatively low energy density of vanadium, redox flow batteries are suited above all for stationary and long-term stable energy storage, such as for electricity generated by wind or solar installations. “Such industrial-scale energy storage plants are urgently needed,” according to Grey, “in order to balance the differences between supply and demand regarding the different sources of renewable energy.” By using NMR studies, her team has already succeeded in observing live the processes that occur in the electrolytes (or liquid fuels) and in recording images (MRI) of them. Grey is furthermore pursuing research on cost-efficient organic electrolytes that do not require poisonous vanadium.
With the funds that accompany the Körber European Science Prize, Grey wants to further refine NMR spectroscopy in the future. This is also the purpose of using DNP technology, during which NMR samples are additionally irradiated with high-energy microwaves. DNP (which stands for dynamic nuclear polarization) is based on the transference of certain quantum mechanical properties of electrons (spin polarisation) to the atom’s nucleus. DNP, according to Grey, “makes it possible to increase the sensitivity of NMR spectrometers by a factor of 1000.”

“One of my most important goals is to convince students, the public, and political decision makers of the fact that the technologies for achieving the zero carbon climate goals by 2050 can only be made available in time if we begin with the basic research now—tomorrow is too late.”
Clare Grey
Fast track to practical applications
Grey is not only active in basic research; she is also interested in a rapid practical application of new battery technologies. The firm Nyobolt, founded in 2019, develops novel rechargeable batteries that have an electrode containing a niobate that was designed by Grey’s lab, which can be recharged in just a few minutes without any danger of fire, and which exhibits a considerably longer lifespan and energy density than conventional lithium-ion rechargeable batteries. The German company NMR Service GmbH, in which Grey also works with, supplies NMR measurement technology developed by the prizewinner to research and industrial laboratories around the world. “One of my most important goals is to convince students, the public, and political decision makers of the fact that the technologies for achieving the zero carbon climate goals by 2050 can only be made available in time if we begin with the basic research now—tomorrow is too late.“
The prizewinner

Clare Grey was born in 1956 the city of Middlesbrough in the northeast of England. When she was two years old, she moved with her parents to the Netherlands. Her father, a chemist, took a position there with the chemical company ICI. Her mother is a doctor of theology.
“As a seven year old, I could speak fluent Dutch,“ Grey says. “Today I unfortunately speak the language worse than then.“ In 1972 her parents moved to Belgium because of work; there she attended a British school. In 1979, when she was 14, her parents moved back to England, where she attended school.
Clare Grey then went on to study chemistry at Oxford University. At the early age of 22, she published her first article in the distinguished science journal “Nature“. Even as an undergraduate, Grey had already worked with NMR spectroscopy and studied metals such as tin. After receiving her doctorate in 1991, she switched to the Radboud University in Nijmegen, The Netherlands: “I was happy to be able to refresh my Dutch again.“ In 1992- 1993 Grey was a visiting scientist at the American chemical company Dupont. In 1994 she became an assistant professor and taught at the State University of New York at Stony Brook, where she was made a regular professor in 2001. In 2009 she became the Geoffrey Moorhouse Gibson Professor at Cambridge University, back in Great Britain. Since 2011 she has also been a Fellow of the Royal Society. In 2019 she was a cofounder of the Nyobolt company, which manufactures rechargeable batteries that charge very rapidly and are reliable.
As a child, Clare Grey wanted most of all to become a physician. Her father gave her a chemistry set, “but the experiments with flames and spraying sparks didn’t inspire me much. And today I know why. I missed the perspective of discovering something new in the experiments.” Later, as a distinguished researcher, she enjoyed “working in completely new fields and being able to ask questions that no one had posed before.” In her leisure time, Grey plays the cello, Brahms for example, and sings. She is an enthusiastic hiker and also plays squash and goes cross-country skiing.

Interview with Tatjana König
Tatjana König on the Körber Prize

“Clare Grey’s application-oriented basic research constitutes a decisive contribution to the development of more powerful storage media. It is only if we are successful in this matter that we can attain CO2 neutrality in the traffic sector and that our chances of achieving our climate goals by 2050 will be at all realistic.”
Tatjana König
Former Member of the Executive Board of Körber-Stiftung
Are science prizes out of step with the time?
The Covid crisis has emphatically confirmed the significance of science, yet it is still not a matter of course for people to listen to scientific reason and to trust it. This makes it all the more important for us to draw attention to the achievements of science and to demonstrate that and how it helps us cope with major social challenges.
And is that also true for this year’s prizewinner?
Absolutely! In the last 18 months, science has achieved fantastic advances in fighting the pandemic, but in the process, we have almost lost track of the fact that we face a few other major challenges whose consequences may probably be longer lasting and even more threatening, most of all the climate crisis. Clare Grey’s application-oriented basic research constitutes a decisive contribution to the development of more powerful storage media. It is only if we are successful in this matter that we can attain CO2 neutrality in the traffic sector and that our chances of achieving our climate goals by 2050 will be at all realistic.
Is there a hidden intention behind having a British prizewinner in the same year as Brexit?
No, our committees are oriented strictly by scientific excellence, but it could well be the cunning of reason. All major crises are now global in nature, and we will only be able to master them through joint efforts. A Europe that is strong and united in science will play a key role here. Independent of political decisions, we maintain for the Körber Prize that Great Britain remains part of Europe, at least as far as science is concerned.
Personally, I am pleased that the prize was awarded to Clare Grey, especially since we at the Körber-Stiftung have resolved to pay more attention to diversity in science. Leading female researchers continue to be in the spotlight much less frequently than their male colleagues. It is all the more rewarding for us to have her as such a great role model!
Award Ceremony 2021
Körber Prize winner Clare Grey in conversation with Ranga Yogeshwar (in German)

Hamburg’s First Mayor Dr. Peter Tschentscher welcomed the guests to the Award Ceremony for the Körber-Preis 2021. 
Dr. Lothar Dittmer, Chairman of the Executive Board of the Körber-Stiftung, referred in his speech to the original idea of the Körber-Preis, that science and research must contribute to improving the living conditions on our planet. 
The scholars of the ‚Deutsche Stiftung Musikleben‘, Annabel Hauk und Anna Olivia Amaya Farias, provided the musical entertainment. 
Ranga Yogeshwar in conversation with Prof. Dr. Clare Grey. 
Ranga Yogeshwar in conversation with Prof. Dr. Clare Grey. 
Ranga Yogeshwar in conversation with Tatjana König and Prof. Dr. Martin Stratmann. 
The Award Ceremony took place in 2021 in compliance with corona measures. 
L-R: Prof. Dr. Martin Stratmann, Tatjana König, Dr. Peter Tschentscher, Prof. Dr. Clare Grey, Dr. Lothar Dittmer. 
L-R: Dr. Lothar Dittmer, Prof. Dr. Clare Grey, Prof. Dr. Martin Stratmann on stage.
Photos of the presentation of the Körber European Science Prize 2021 to Clare Grey in the Hamburg City Hall on 10 September 2021. These photos are free to use in the context of news coverage with the credits given below.
New Batteries for more Climate Protection
Learn more about Clare Grey and her prizewinning breakthrough.